16 Sep “Update in Understanding and Managing FEVR” published in Retina Today, featuring Dr. Kimberly A. Drenser, MD, PhD
Update in Understanding and Managing FEVR
New research approaches and potential therapeutics are moving the field toward improved care for these patients.
By Kimberly A. Drenser, MD, PhD
April 2022
Familial exudative vitreoretinopathy (FEVR) is a relatively rare hereditary disease that remains poorly understood. Although there’s a lack of published data on its prevalence in the United States and worldwide, preliminary research in my tertiary referral group suggests that FEVR meets the US FDA’s criteria of a rare disease (affecting fewer than 200,000 people in the United States). Of course, gathering data from a tertiary referral center may introduce some bias into the prevalence. Recent research advances have improved our knowledge of FEVR disease pathology, leading to a better understanding of the prognosis and the effect of various management strategies.
Management for FEVR has matured significantly over the last 20 years with improved tools and how aggressively we use them, depending on the case. Some types of FEVR are relatively mild and remain so with control of the initial complications. Other types, however, are unrelenting and require aggressive management and frequent evaluation.
In this article, I review what we currently know about FEVR, how we are managing it in the clinic, and what the future holds.
BLOOD-RETINA BARRIER BREAKDOWN
Much of the management is based on our understanding of the pathways and genes that cause FEVR. In the eye, the Wnt signaling pathway regulates retinal development, maintenance, and repair.1,2 This norrin-driven pathway promotes the development of normal capillary beds with nonfenestrated vessels, and when it breaks down in FEVR, those capillary beds are impaired. This leaves an underdeveloped eye and creates a large area of avascular peripheral retina; more recent evaluations, particularly with OCT angiography, have shown that the intermediate and deep capillary beds are also impaired to varying degrees.3,4
With an impaired Wnt pathway, all forms of FEVR exhibit some degree of blood-retina barrier breakdown; some will have very poor tight junctions, ischemic drive, and continued exudation, while others also may have frank bleeding and neovascularization.
TREATMENT APPROACHES
All of today’s FEVR interventions focus on managing and slowing progression of disease and vision loss—they don’t directly address the underlying issue of incomplete retinal development and lack of vascularization of normal retina. By definition, these patients are constantly progressing and losing vision, whether minimally or aggressively.
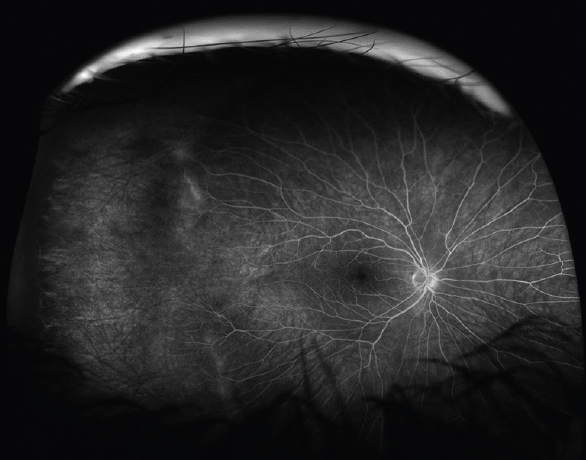
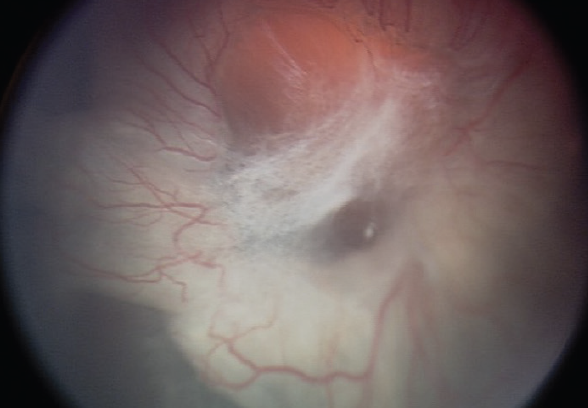
Eyes with severe forms of FEVR often require frequent anti-VEGF treatment to reduce the excessive VEGF drive due to an avascular retina (Figure 1) and capillary dropout (Figure 2), and to minimize the tight junction breakdown. Milder presentations may be managed with laser ablation of the avascular retina. Once patients fall into a pattern of severe exudation, similar to patients with diabetes, they also experience an inflammatory response, limiting the utility of anti-VEGF therapy. However, if the patient has chronic and excessive exudation due to that tight junction breakdown, adding steroids to the treatment regimen is another additive therapeutic. The most aggressive solution is surgery, usually reserved for patients who have a substantial amount of scar tissue and/or tractional changes, which almost always requires a vitrectomy to address surface proliferation and transvitreal adhesions and contractions. Scleral buckling has less utility in these cases with the exception of combined rhegmatogenous/tractional detachments (Figure 3) or to reduce severe anterior vitreous base contraction.
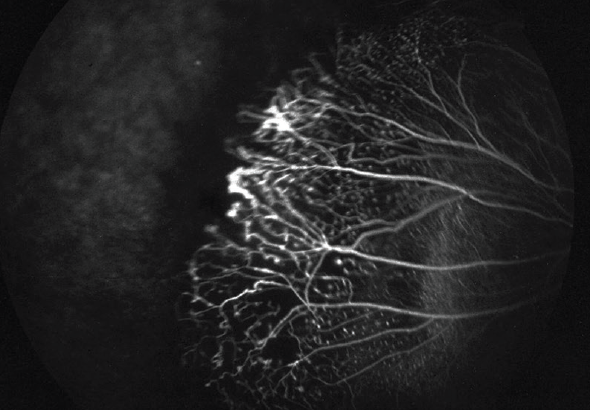
FEVR also comes with concerns for hypoxia, which can be addressed with early laser treatments. Clinicians can laser ablate the avascular retina and monitor for continued capillary loss. If capillary beds continue to drop out, retreatment with laser can help to control the ischemia. Patients who achieve ischemic control usually remain relatively stable.5
NEXT STEPS
Clinicians and researchers are now focusing on not just controlling the symptoms of FEVR but also regrowing those capillary beds with an intact blood-retina barrier. New therapeutics, still in the early preclinical stage, may one day help to reestablish a normal growth pattern in a child or young adult after retinal development is complete, such as Caeregn Therapeutics’ (a company Antonio Capone Jr, MD; Michael T. Trese, MD; and I cofounded) product CTR-107 (Noregen).
Early proof-of-concept studies in animal models have shown that new approaches can regenerate nonfenestrated capillaries in the retina, which also increases the retinal ganglion cell count.6,7
CTR-107 is a recombinant modified norrin mimic. Cell-based data looking at gene expression is another hopeful area of research that may be able to validate that targeting the Wnt/norrin-driven pathway of rescue is working. These cell-based assays allow for gene expression analysis and downstream pathway activation targets that validate the norrin-mediated Wnt signaling.
The biggest area of research is product characterization, including protein mapping, mass spectrometry, and other analytics to clearly characterize the new potential products and validate them for human use.
Investigating treatments for rare diseases is a challenge, and having a concentrated number of FEVR patients in my practice is what has enabled me and my team to improve our management and understanding of FEVR, initiate pilot studies in research, and translate that research into a potential therapeutic agent.
REFERENCES
1. Warden SM, Andreoli CM, Mukai S. The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol. 2007;22(4):211-217.
2. Drenser KA. Wnt signaling pathway in retinal vascularization. Eye Brain. 2016;8:141-146.
3. Koulisis N, Moysidis SN, Yonekawa Y, et al. Correlating changes in the macular microvasculature and capillary network to peripheral vascular pathologic features in familial exudative vitreoretinopathy. Ophthalmol Retina. 2019;3(7):597-606.
4. Chen C, Liu C, Wang Z, et al. Optical coherence tomography angiography in familial exudative vitreoretinopathy: clinical features and phenotype-genotype correlation. Invest Ophthalmol Vis Sci. 2018;59(15):5726-5734.
5. Ranchod TM, Ho LY, Drenser KA, Capone A Jr, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070-2075.
6. Tokunaga CC, Chen YH, Dailey W, Cheng M, Drenser KA. Retinal vascular rescue of oxygen-induced retinopathy in mice by norrin. Invest Ophthalmol Vis Sci. 2013;54(1):222-229.
7. Dailey WA, Drenser KA, Wong SC, et al. Norrin treatment improves ganglion cell survival in an oxygen-induced retinopathy model of retinal ischemia. Exp Eye Res. 2017;164:129-138.
Kimberly A. Drenser, MD, PhD
Partner, Associated Retinal Consultants, Royal Oak, Michigan
Professor, Department of Ophthalmology, William Beaumont Oakland University School of Medicine, Rochester, Michigan
Director, Pediatric Retinal Research Laboratory, Eye Research Institute at Oakland University, Rochester, Michigan
Editorial Advisory Board Member, Retina Today
[email protected]
Financial disclosure: Cofounder/Intellectual Property (Caeregen Therapeutics)
