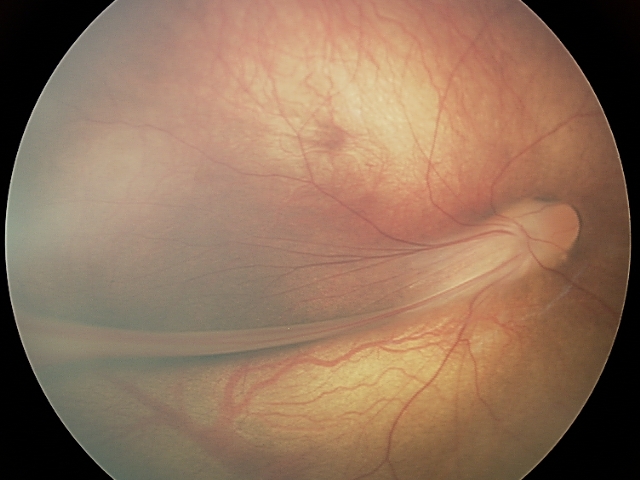
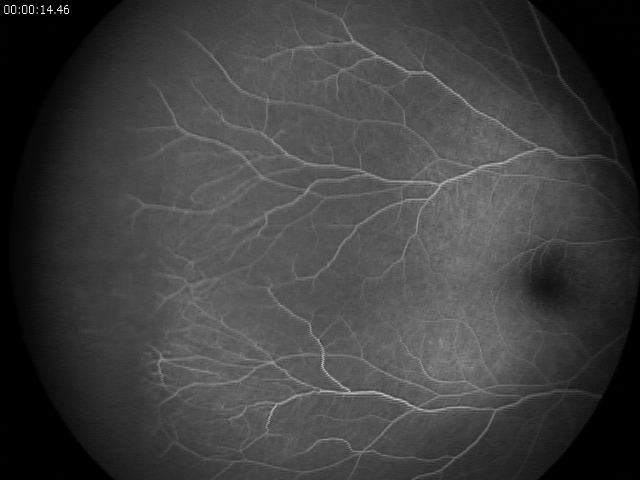
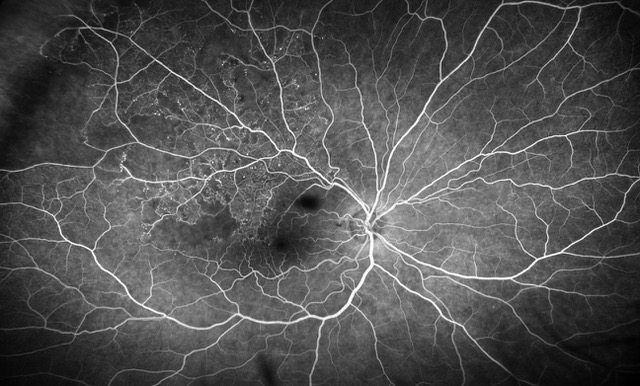
FEVR is a life-long hereditary retinal vascular disease. Impaired angiogenesis in the retina of young children can lead to aberrant neovascularization and symptoms, such as the formation of retinal folds and tears, retinal detachment, or even total vision loss.1 Among the genes associated with FEVR, components of the Wnt/β-catenin signaling pathway are highly prevalent. These are the genes encoding the growth factor Norrin (NDP), the receptor Frizzled-4 (FZD4), the low-density lipoprotein receptor-related protein (LRP5), and tetraspanin-12 (TSPAN12), which forms transmembrane receptorcomplexes with LRP5 and Frizzled-4.
Familial exudative vitreoretinopathy (FEVR) was first described by Criswick and Schepens.2 It is a rare disorder of retinal blood vessel development principally affecting retinal angiogenesis, leading to incomplete vascularization of the peripheral retina and poor vascular differentiation. Cases can be inherited in an autosomal dominant, autosomal recessive, or X-linked manner, or can affect individuals with no family history. Most FEVR patients have an avascular peripheral retina but expressivity may be asymmetric and is highly variable, ranging from asymptomatic to severe within the same family. If there is a significant degree of retinal ischemia, then secondary neovascularization can occur leading to fibrosis, traction of the posterior pole structures, retinal detachment (RD), retinal folds, or complete retinal dysplasia in the most severe cases. A diagnosis of FEVR can be made if there is evidence of peripheral retinal avascularity in Z1 eye in patients of any age who were born at full term, or preterm with a disease tempo not consistent with retinopathy of prematurity (ROP).
Five genes have so far been identified that when mutated, cause FEVR; NDP (MIM 300658), FZD4 (MIM 604579), LRP5 (MIM 603506), TSPAN12 (MIM 613138), and ZNF408. Mutations in these genes account for around 50% of FEVR cases.3 Four of the genes identified to date have been shown to have a role in a variant of the Wnt signaling pathway, termed as Norrin/Frizzled4 signaling suggesting a crucial role for this pathway in retinal vascular development.
Currently however, there are no approved pharmacological therapies for FEVR.
1 Park, H., Yamamoto, H., Mohn, L. et al. Integrin-linked kinase controls retinal angiogenesis and is linked to Wnt signaling and exudative vitreoretinopathy. Nat Commun10, 5243 (2019). https://doi.org/10.1038/s41467-019-13220-3
2, 3 Gilmour, D. Familial exudative vitreoretinopathy and related retinopathies. Eye 29, 1–14 (2015). https://doi.org/10.1038/eye.2014.70